In our aging population, large joint replacements and implantable cardiac devices are becoming increasingly prevalent. For instance, it is estimated that by 2020, almost 30% of the 65+ population in five major countries (France, Germany, Italy, Spain and the UK) in Europe will have one or more cardiac or orthopedic implants. These implants limit the possibility of doing MRI, reducing the diagnostic options for those patients.1 This affects diagnostics in oncology as well, for instance for prostate cancer, a disease with patients that are typically 65+. In clinical practice, scanning patients with implants brings challenges. It can be a real puzzle to identify the implant and figure out the condition limits that need to be applied when scanning the patient. As a result, patients with implants are often denied an MRI scan. However, for many of these patients, MRI scans do not have to be ruled out. Philips ScanWise Implant guides the user through simple steps to enter MR parameter values of the important conditions.
The risks of medical implants for MRI
An important step in working with MR Conditional implants is reducing the uncertainty and the time that is needed to find the exact Manufactures conditions required. Once you have identified the implant and found the associated implant manufactures conditional values, the Philips ScanWise* Implant software provides step-by-step guidance to enter the condition values of the implant as specified by its manufacturer. With ScanWise Implant, the system automatically adjusts al scan and pre-scans parameters to meet the implant conditions entered by the user. ScanWise Implant not only helps enhance your confidence in dealing with MR Conditional implants, it also saves time. The step-by-step approach helps reduce guesswork and calculations. The automatic scan parameter adjustment means that you only have to enter the conditions once for an entire exam. And the guided user interface helps you enter the implant conditions in three minutes or less. *Disclaimer: Initial release 1.5T.
ScanWise streamlines working with patients who have MR-conditional implants
An important step in working with MR-conditional implants is reducing the uncertainty and the time that is needed to find the exact conditions required. Once you have identified the implant, the Philips ScanWise Implant software provides step-by-step guidance to enter the condition values of the implant as specified by its manufacturer. ScanWise Implant then automatically adjusts the scan parameters for all scans and pre-scans throughout the examination.
ScanWise Implant not only helps enhance your confidence in dealing with MR-conditional implants, it also saves time. The step-by-step approach helps reduce guesswork and calculations. The automatic scan parameter adjustment means that you only have to enter the conditions once for an entire exam. And the guided user interface helps you enter the implant conditions in three minutes or less.
Meeting a growing need
The need for an MRI increases with age2. The same is true of the prevalence of medical implants. Philips ScanWise Implant supports clinicians in offering MRI scans to patients with MR Conditional implants with more confidence and ease. This allows your hospital to serve a growing population of patients who would otherwise be limited in their diagnostic and MR simulation options.
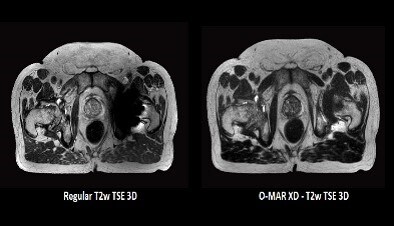
O-MAR XD ** as acquired on Ingenia MR-RT 1.5T on a patient with a uni-lateral hip implant.
An excellent complement to ScanWise Implant is the O-MAR metal artifact reduction algorithm. An MR Conditional implant could still limit the use of the MR image, in cases where the area of interest is close to the implant. In a prostate cancer patient with a hip replacement, for instance, the prostate and critical structures like the seminal vesicles may be blocked by the signal void. O-MAR** improves visualization of soft tissue and bone in the vicinity of the implant. For CT imaging, O-MAR has shown its added value in streamlining the workflow in contouring target volumes and critical structures as part of simulation and patient marking in radiation therapy (see the earlier Hot Spot article).
For more information
1 Philips, data on file. Based on Millennium research group reports RPUS21LJ09; RPUS21LJ10; RPUS21LJ15; RPEU21LJ08; RPEU21LJ15; RPUS50DI14; RPUS20SP10; RPEU20SP10; RPUS21LJ10; RPEU20SP13; RPUS20SP15; US12CR07; RPGL12CR10; RPGL12CR14 and Barmer GEK Arztreport 2011. **Only for use with MR Safe or MR Conditional implants by strictly following the Instructions For Use.
2 Reference: Philips, data on file. Based on Millennium research group reports RPUS21LJ09; RPUS21LJ10; RPUS21LJ15; RPEU21LJ08; RPEU21LJ15; RPUS50DI14; RPUS20SP10; RPEU20SP10; RPUS21LJ10; RPEU20SP13; RPUS20SP15; US12CR07; RPGL12CR10; RPGL12CR14 and Barmer GEK Arztreport 2011.
Written by:

Marieke van Grootel Product Marketing Manager MR Therapy
